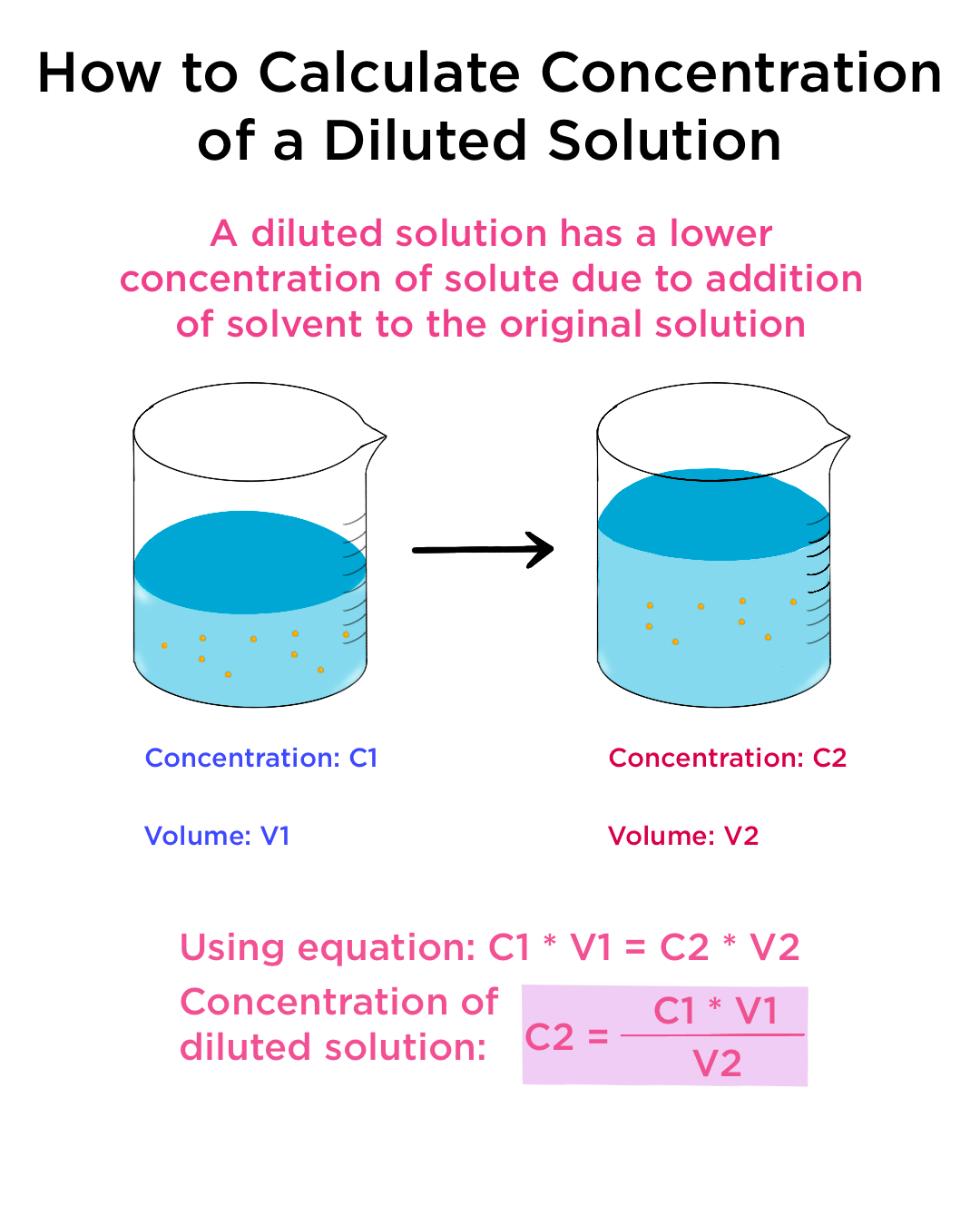
Dilution Formula Explanation . The property that the amount of solute does not change on dilution and concentration gives us a way. C 1 v 1 = c 2 v 2 where:. C 1 v 1 = c 2 v 2 where: apply the dilution equation to calculate the final concentration, or the final volume, of a diluted solution. dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the. Once you understand the above relationship, the. preparing dilutions is a common activity in the chemistry lab and elsewhere. to make a fixed amount of a dilute solution from a stock solution, you can use the formula: V 1 = volume of. to make a fixed amount of a dilute solution from a stock solution, you can use the formula:
from www.expii.com
apply the dilution equation to calculate the final concentration, or the final volume, of a diluted solution. preparing dilutions is a common activity in the chemistry lab and elsewhere. Once you understand the above relationship, the. to make a fixed amount of a dilute solution from a stock solution, you can use the formula: C 1 v 1 = c 2 v 2 where: to make a fixed amount of a dilute solution from a stock solution, you can use the formula: dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the. V 1 = volume of. C 1 v 1 = c 2 v 2 where:. The property that the amount of solute does not change on dilution and concentration gives us a way.
Dilution of Solutions — Overview & Examples Expii
Dilution Formula Explanation V 1 = volume of. to make a fixed amount of a dilute solution from a stock solution, you can use the formula: The property that the amount of solute does not change on dilution and concentration gives us a way. to make a fixed amount of a dilute solution from a stock solution, you can use the formula: Once you understand the above relationship, the. dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the. preparing dilutions is a common activity in the chemistry lab and elsewhere. apply the dilution equation to calculate the final concentration, or the final volume, of a diluted solution. C 1 v 1 = c 2 v 2 where:. V 1 = volume of. C 1 v 1 = c 2 v 2 where:
From www.youtube.com
Dilution formula and how to use it to solve problems. 3 examples solved Dilution Formula Explanation The property that the amount of solute does not change on dilution and concentration gives us a way. preparing dilutions is a common activity in the chemistry lab and elsewhere. dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the. Once you understand the above relationship, the.. Dilution Formula Explanation.
From www.ck12.org
Dilution (M[i]V[i]=M[f]V[f]) Overview ( Video ) Chemistry CK12 Dilution Formula Explanation to make a fixed amount of a dilute solution from a stock solution, you can use the formula: dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the. Once you understand the above relationship, the. The property that the amount of solute does not change on dilution. Dilution Formula Explanation.
From www.slideserve.com
PPT Chapter 10 Acids and Bases PowerPoint Presentation, free download Dilution Formula Explanation Once you understand the above relationship, the. apply the dilution equation to calculate the final concentration, or the final volume, of a diluted solution. to make a fixed amount of a dilute solution from a stock solution, you can use the formula: C 1 v 1 = c 2 v 2 where: C 1 v 1 = c. Dilution Formula Explanation.
From www.youtube.com
DILUTION AND MIXING OF SOLUTIONS, MOLARITY EQUATION /SOME BASIC Dilution Formula Explanation Once you understand the above relationship, the. dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the. to make a fixed amount of a dilute solution from a stock solution, you can use the formula: C 1 v 1 = c 2 v 2 where: to. Dilution Formula Explanation.
From www.youtube.com
Chem 11 Dilution Calculations_Solving for Final Volume after Dilution Dilution Formula Explanation V 1 = volume of. preparing dilutions is a common activity in the chemistry lab and elsewhere. C 1 v 1 = c 2 v 2 where: The property that the amount of solute does not change on dilution and concentration gives us a way. dilution is the process of “lowering the concentration of a solute in a. Dilution Formula Explanation.
From exoubqlmz.blob.core.windows.net
Dilution Equation With at Sharon Firestone blog Dilution Formula Explanation V 1 = volume of. The property that the amount of solute does not change on dilution and concentration gives us a way. C 1 v 1 = c 2 v 2 where: to make a fixed amount of a dilute solution from a stock solution, you can use the formula: dilution is the process of “lowering the. Dilution Formula Explanation.
From www.youtube.com
Dilution and Dilution Factor in Microbiology How to Calculate Dilution Formula Explanation C 1 v 1 = c 2 v 2 where: apply the dilution equation to calculate the final concentration, or the final volume, of a diluted solution. to make a fixed amount of a dilute solution from a stock solution, you can use the formula: The property that the amount of solute does not change on dilution and. Dilution Formula Explanation.
From www.youtube.com
TRU Chemistry Labs How To do Dilution Calculations YouTube Dilution Formula Explanation preparing dilutions is a common activity in the chemistry lab and elsewhere. C 1 v 1 = c 2 v 2 where:. Once you understand the above relationship, the. to make a fixed amount of a dilute solution from a stock solution, you can use the formula: apply the dilution equation to calculate the final concentration, or. Dilution Formula Explanation.
From www.youtube.com
Dilution in biology lab How to use the equation C1V1 = C2V2 YouTube Dilution Formula Explanation preparing dilutions is a common activity in the chemistry lab and elsewhere. dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the. to make a fixed amount of a dilute solution from a stock solution, you can use the formula: apply the dilution equation to. Dilution Formula Explanation.
From www.slideshare.net
Molarity and dilution Dilution Formula Explanation preparing dilutions is a common activity in the chemistry lab and elsewhere. C 1 v 1 = c 2 v 2 where: dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the. apply the dilution equation to calculate the final concentration, or the final volume, of. Dilution Formula Explanation.
From www.slideserve.com
PPT Chapter 8 Solutions PowerPoint Presentation, free download ID Dilution Formula Explanation C 1 v 1 = c 2 v 2 where: The property that the amount of solute does not change on dilution and concentration gives us a way. V 1 = volume of. dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the. preparing dilutions is a. Dilution Formula Explanation.
From www.scientistcindy.com
Dilution Series and Calculations SCIENTIST CINDY Dilution Formula Explanation preparing dilutions is a common activity in the chemistry lab and elsewhere. apply the dilution equation to calculate the final concentration, or the final volume, of a diluted solution. Once you understand the above relationship, the. dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the.. Dilution Formula Explanation.
From www.youtube.com
How to Calculate Dilution Factor YouTube Dilution Formula Explanation to make a fixed amount of a dilute solution from a stock solution, you can use the formula: C 1 v 1 = c 2 v 2 where: C 1 v 1 = c 2 v 2 where:. dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to. Dilution Formula Explanation.
From www.slideserve.com
PPT Chapter 1 The Organization of Matter PowerPoint Presentation Dilution Formula Explanation V 1 = volume of. to make a fixed amount of a dilute solution from a stock solution, you can use the formula: Once you understand the above relationship, the. apply the dilution equation to calculate the final concentration, or the final volume, of a diluted solution. dilution is the process of “lowering the concentration of a. Dilution Formula Explanation.
From study.com
Dilution Definition, Equation & Factors Video & Lesson Transcript Dilution Formula Explanation C 1 v 1 = c 2 v 2 where: Once you understand the above relationship, the. to make a fixed amount of a dilute solution from a stock solution, you can use the formula: V 1 = volume of. to make a fixed amount of a dilute solution from a stock solution, you can use the formula:. Dilution Formula Explanation.
From www.expii.com
Dilution of Solutions — Overview & Examples Expii Dilution Formula Explanation to make a fixed amount of a dilute solution from a stock solution, you can use the formula: dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the. preparing dilutions is a common activity in the chemistry lab and elsewhere. The property that the amount of. Dilution Formula Explanation.
From www.youtube.com
Chem143 Dilution Equation YouTube Dilution Formula Explanation apply the dilution equation to calculate the final concentration, or the final volume, of a diluted solution. preparing dilutions is a common activity in the chemistry lab and elsewhere. dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the. C 1 v 1 = c 2. Dilution Formula Explanation.
From chemistnotes.com
Isotopic Dilution Method Detailed Explanation Chemistry Notes Dilution Formula Explanation dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the. Once you understand the above relationship, the. preparing dilutions is a common activity in the chemistry lab and elsewhere. V 1 = volume of. apply the dilution equation to calculate the final concentration, or the final. Dilution Formula Explanation.